123 Main Street, New York, NY 10001
The Co-Dx Logix Smart® SARS-CoV-2 (genes RdRp/E) test kit is an in vitro diagnostic test that uses our patented Co-Primers™ technology for the qualitative detection of two genes (gene RdRp and gene E) from the RNA of SARS-CoV-2 (coronavirus that causes COVID-19).
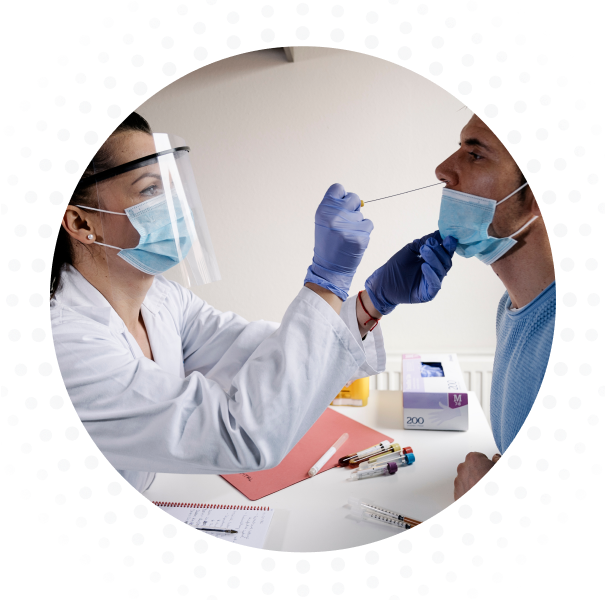
The test operates using a single step real-time reverse transcriptase PCR process in lower respiratory tract fluids (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract fluids (e.g. nasopharyngeal and oropharyngeal swabs), and saliva during the acute phase of detection.
Click here to read the Company’s regulatory bulletin on the ability of our suite of Co-Dx Logix Smart COVID-19 diagnostics to detect all known strains of SARS-CoV-2.
The Co-Dx Logix Smart SARS-CoV-2 (genes RdRp/E) test kit includes the following components.
The Co-Dx Logix Smart SARS-CoV-2 (genes RdRp/E) test kit is a real-time PCR multiplex test intended for the in vitro qualitative detection of nucleic acid from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), targeting the genes RdRp in the polygene Orf1ab region and gene E of the virus genome, in lower respiratory tract samples (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract samples (e.g. nasopharyngeal and oropharyngeal swabs), and saliva from individuals suspected of COVID-19.
The Co-Dx Logix Smart SARS-CoV-2 (genes RdRp/E) test kit is intended for use by trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
sbeadex Viral RNA Pruification Kit
automated with oKtopure High Throughput system (both LGC Biosearch Technologies)
The test should work with most qPCR systems with the following channel compatibilities:
QIAamp® Viral RNA Mini Kit (Qiagen, CAT #52904, 52906)
HighPrep Viral DNA/RNA (MagBio Genomics, CAT #HPV-DR96
Viral DNA/RNA Kit (CW Bio. CAT #CW3126M)
sbeadex Viral RNA Purification Kit (LGC Biosearch Technologies CAT #NAP-40-026-04).
* Results obtained from observational study of 2364 datapoints obtained from the contrived samples
Contact us for more information about the Co-Dx Logix Smart SARS-CoV-2 (genes RdRp/E) test.
Every test from Co-Dx is designed to be easy to use.
In November 2020, the Co-Dx Logix Smart SARS-CoV-2 (genes RdRp/E) test kit received CE marking approval, the principle regulatory clearance allowing the test to be sold as an in vitro diagnostic (IVD) for the diagnosis of COVID-19 in European Union states and other markets that accept a CE-IVD mark as valid regulatory approval.
Is this test FDA-approved or cleared?
No. This test is not yet approved or cleared by the U.S. Food and Drug Administration (FDA). This product is not available for sale within the United States.
Co-Diagnostics, Inc.
2401 S. Foothill Dr. Suite D
Salt Lake City, UT 84109 USA
1 (801) 438-1036
info@co-dx.com
Co-Dx, Co-Primers, Logix Smart, Co-Dx Box, PCR Home, PCR Pro, and Vector Smart, and unless otherwise specified, all other product and service names appearing in this Internet site are trademarks owned by Co-Diagnostics, Inc., its subsidiaries or affiliates. No use of any Co-Diagnostic trademark, trade name, or trade dress in this site may be made without the prior written authorization of Co-Diagnostics, except to identify the product or services of the company.
TaqMan is a trademark of Roche Molecular Systems.